Micro and nanobubbles are tiny gas-filled bubbles that are extensively used in various applications such as biomedical imaging, drug delivery, and water treatment. Free radicals are highly reactive chemical species that can cause damage to biological molecules such as lipids, proteins, and DNA. Free radicals are generated in micro and nanobubbles due to various mechanisms, including physical and chemical processes.
One of the physical processes that generate free radicals in micro and nanobubbles is cavitation. Cavitation is the formation and collapse of gas-filled bubbles in a liquid medium due to changes in pressure. When a micro or nanobubble is exposed to an ultrasound wave, it undergoes expansion and contraction cycles that lead to cavitation. During the collapse phase of the bubble, a high-pressure zone is created that generates free radicals in the surrounding liquid. These free radicals can be generated due to various mechanisms, such as the dissociation of water molecules, fragmentation of organic compounds, and production of reactive oxygen species.
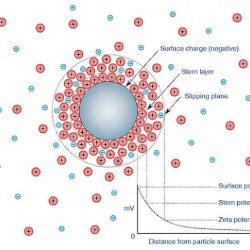
Chemical processes can also generate free radicals in micro and nanobubbles. For instance, when micro or nanobubbles are in contact with a surface, such as a biological membrane or a solid support, they can undergo chemical reactions that lead to the production of free radicals. In particular, the gas-liquid interface of micro and nanobubbles provides a favorable environment for the production of free radicals through redox reactions. For example, when oxygen molecules are dissolved in the liquid phase, they can react with electrons on the bubble surface, generating reactive oxygen species such as superoxide and hydroxyl radicals.
In summary, free radicals can be generated in micro and nanobubbles through physical and chemical processes, including cavitation, redox reactions at the gas-liquid interface, and other chemical reactions. The production of free radicals in micro and nanobubbles can have both positive and negative effects on their applications, depending on the intended use and the conditions in which they are employed