This review article organizes the studies conducted on the areas of microbubbles and nano bubbles with a special emphasis on water treatment. The basic definitions of bubble types and their size ranges are also presented based on the explanations of different researchers. The characterization parameters with state-of-the-art measuring and analysis techniques of micro-bubble and nanobubble technologies are summarized. Some major applications of these technologies in water-treatment processes are reviewed and briefly discussed. Based on the reviews, various potential areas for research and bubble application gaps in water and wastewater treatment technologies are identified for further study. The article is prepared in such a way that it provides a step-by-step acquaintance to the subject matter with the objective of focusing on the application of microbubbles and nano bubbles in water-treatment technology.
Operations that involve phase changes are largely applicable in environmental, agricultural, healthcare, chemical or industrial and energy fields. Among the phase-related processes, gas–liquid operations are often applied in wastewater and water treatment technologies, aquatic system restoration, food processing industries, aqua farming, and chemical as well as petrochemical industries. The efficiency of most gas–liquid processes are affected by different design and operational parameters, types of contacting operations, and the properties of contacting fluids. In the basic theoretical phase mass-transfer theories, an increase in the contact-surface area is one of the major factors that contribute towards an improvement in the process performance. Moreover, mixer design, packing column materials, tray and baffle type structures, spray methods, injection nozzles, and sparger-type designs, are other parameters that play an important role in enhancing either the heat and mass transfer or reaction-surface areas between the contacting phases.
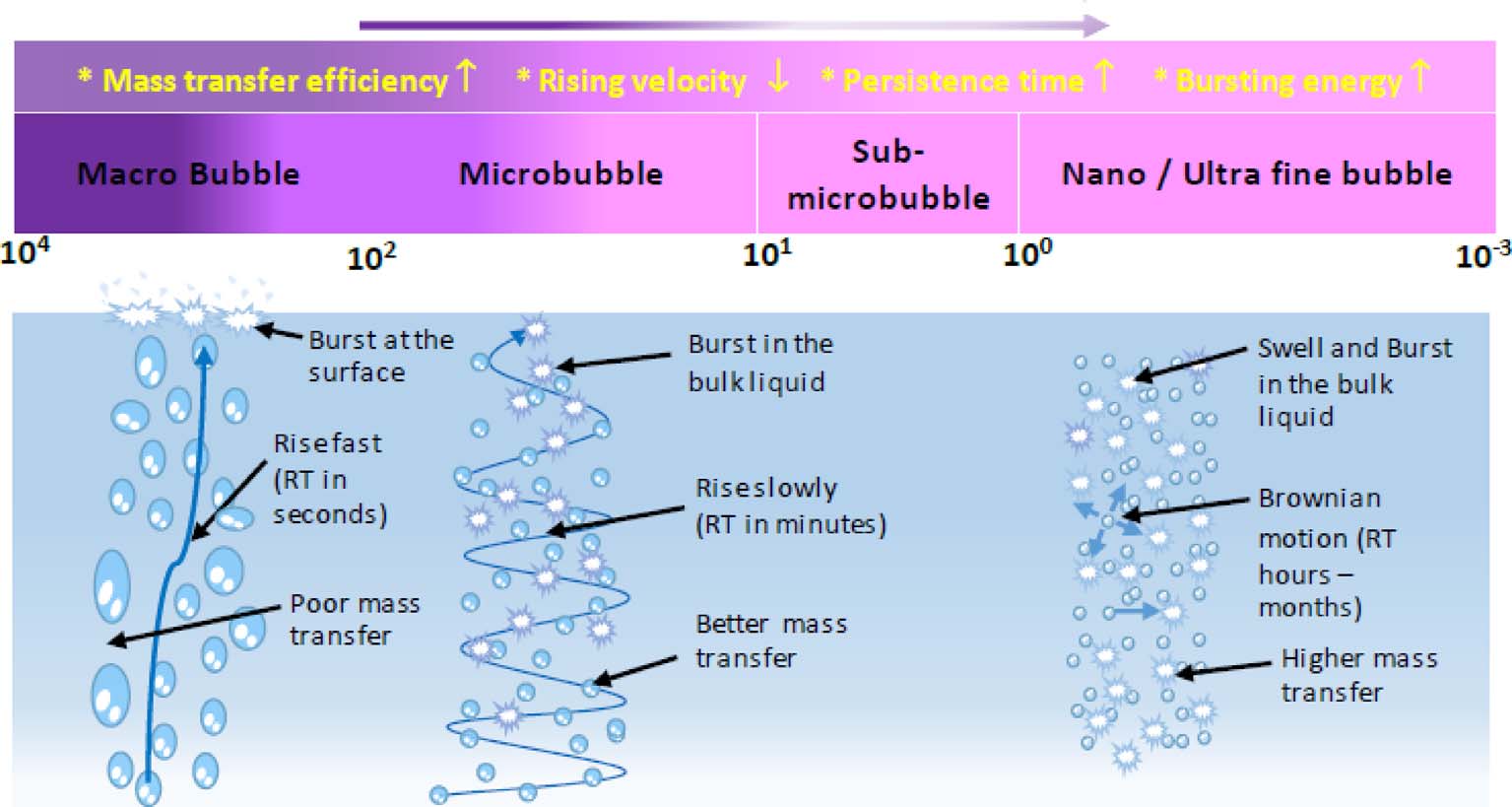
In the past two decades, many researchers have been focusing on microbubbles (MBs) and nano bubbles (NBs) as a way of improving the efficiency of gas–liquid phase processes. Most studies focused on the generation methods, measuring techniques, and characterization of fine and ultra fine bubble categories. Recently, few studies revealed the possibility of scale-up and application of MB and NB application at industrial levels. Based on some preliminary results, most researchers suggested that MB and NB applications in water-treatment technologies have great potential.
Limited efforts have been made on collecting and preparing a well organized, comprehensive report of the researches done in the areas of MBs and NBs with respect to water-treatment technologies. In addition, existing studies have some discrepancies and vague boundaries, particularly in defining the range of bubble category. In this comprehensive review, extensive effort was made to reduce such discrepancies and provide concise information regarding the current condition of MB and NB applications in water treatment. In the review, a step wise approach was followed while addressing general to specific issues of MB and NB applications in water-treatment technologies. Different types of bubbles are defined, and a range of bubble sizes is proposed for each type based on the common properties presented in different studies. The general MB and NB generation techniques that were previously proposed are summarized to discuss the major bubble characterization parameters, existing measurement methods and characterization techniques. The focus of this review is to extensively summarize the current status of MBs and NBs in water-treatment technologies, reveal existing research gaps and address future application scope of this technology in the field.
Application of MBs and NBs in water-treatment technology has been recently gaining attention. It has been reported that MBs and NBs can detoxify contaminated water by catalyzing the reactions of chemicals, thereby improving the efficiency of the chemical treatment process. According to several studies, bubbles generated from air and nitrogen could enhance the activities of microorganisms and/or bacteria in both anaerobic and aerobic conditions, in defouling solid surface and membranes. In different literature, MBs and NBs have been applied in typical treatment processes, flotation, and disinfection. The most commonly known processes applied in water treatment utilizing bubble technology are flotation, aeration, disinfection and advanced oxidation processes. In most of the researches done, the main objectives of applying MB and NB technologies are to downsize the facilities, to reduce operation time and reduce operation and maintenance cost of water-treatment plants, with a greater contaminant removal efficiency.
Aeration
In natural water restoration and water treatment, aeration plays a major role in delivering oxygen, which is an important life-sustaining component for aquatic lives and biochemical reaction substrate in aerobic treatment. Moreover, fluid therapy is one of the most common intervention areas in cell biology and healthcare. Many studies have been conducted on the effect of aeration processes in biological water and wastewater treatment, ground water remediation as well as agriculture. The main target objectives of most studies are improving the aeration efficiency to improve biological matter degradation, microbial growth rate, seed germination, and growth rates. In the previous studies, the main concern is optimizing the efficiency of the mass transfer rate-limiting factor. In the conventional aerobic systems, dissolved oxygen is a critical factor to consider in efficiency. In these conventional systems, the mass-transfer rate of oxygen is very important because most contacting equipment utilize the mechanical aerators or diffusers, which require high electrical input and mechanical part maintenance resulting in high operating costs. The attention of most of the aeration researches more focused on the design of aerators and optimization of operating parameters for conventional bubbles.
Recently application of MBs and NBs received attention in the processes stated earlier to improve mass transfer in the area of aeration. But, researches in the application of this bubble types at the industrial scale is not yet well developed, studied the dispersion and gas– liquid mass-transfer characteristics of the MBs for aeration in stirred tank reactors and concluded that a higher impeller speed does not always mean a greater kla, and proposed that the MB aeration was more suitable for bioreactors, investigated the impact of the MB aeration on the fermentation of Trichoderma reesei, which is highly affected by the limited mass-transfer rate of oxygen. Their study concluded that, by applying the MB aeration, the dissolved oxygen concentration was above the critical concentration at low agitation rates and the cell mass concentration increases very rapidly during the fast growth stage with the mass productivity increment from 0.1 to 0.18 g/lh in comparison to the conventional bubbling. In another research studied the oxidative capacity of the NB aeration of seeds and its effects on germination. Their result concluded that the seed germination was improved by using the NB aerated water in comparison to those submerged in distilled water. Similarly, investigated the effect of the MB aeration on the growth of leaf lettuce (Lactuca sativa) and found that fresh and dry weights of the MB aerated lettuce are 2.1 and 1.7 times greater than the macro bubble aerated lettuce, respectively. In both studies, the researchers speculated that the resulted germination and growth improvement was because of the high specific-surface area of Micro-NBs and the ability of attracting positive ions owing to their high negative electronic charges. However, the effect of MBs and NBs on nitrifying bacteria and other useful bacteria growth was overlooked, studied the aeration possibility of ground water by micro-NBs and found that the hydraulic conductivity of the sand was not affected by the micro-NBs inside the pore water. Moreover, the mass transfer rate of oxygen to the water was found to be 125 times faster than the macro bubbled water which was 16 times less dissolved oxygen enhancement durability compared to micro-nano-bubbled water. Based on the outcome of the study, they suggested that the applications of MBs and NBs can predictably facilitate the aerobic bio degradation process for ground-water contaminant removal. Considering the limitation of Micro-NBs application studies in wastewater treatment the authors of this paper performed research on the utilization of NBs for aerobic waste degradation. The results show that the oxygen utilization rate and the volumetric mass transfer coefficient in the NB aerated synthetic wastewater treatment unit is almost double compared to a conventional bubble aerated one. The organic waste degradation in the NB aerated unit took less than half of the retention time used for the conventional system. Similarly, the biomass system in the NB aerator unit showed higher growth and decay rates. Based on the experience of our results it is highly recommended to work on further tests and researches in this area.
Flotation
Flotation had been one of the basic separation processes in water treatment for many decades. Most of the target substances to be separated by flotation include powders, chemicals, metal ions, oils and organic (Rubio et al., 2002). The need in reduction of bubble size in flotation processes is highly related to improving separation efficiency, explained the possibility of optimizing the efficiency of flotation by modification of bubble characteristics mainly size and surface charge in addition to particle properties. From the perspective of flotation, both the MBs and NBs are widely used to remove the contaminants in water with a better efficiency. There is a large body of experimental evidence showing that bubble–particle collection efficiency, investigated the effect of bubble size reduction to MB on the rate of fine particles. The bubble size was controlled by varying impeller speed. They found out that the flotation rate was strongly affected by bubble size to the extent of one hundredfold for a bubble size reduction from 655 μm to 75 μm. Similarly, reported the need of bubble size reduction in froth flotation is highly related to increasing the collusion probability of small particles with fine gangue. According to the study the probability of collusion increases as the bubble size decreases improving the separation efficiency. Surface charge and size of particles as well as small bubbles play an important role in flotation. It has been provided that positively charged MBs could efficiently separate algae from freshwater, with approximately 90% of cell number removal and 92% of chlorophyll a reduction. For organic matter, i.e., total organic carbon and dissolved organic carbon and other aliphatic/aromatic compounds, a removal rate >30% could be achieved in the application of the MBs. The principle for removal mechanism of the contaminants in water is attributed to the similar size and opposite charge between the bubbles and fine particles, which leads to better collision of bubbles and contaminated particles, making it easy to float. Increase in concentration of NB in froth flotation increases the stability of MB suspension reducing rising velocity creating favorable condition for froth flotation of coarse particles or hard-to-float particles that require a relatively long slide time. In another study studied the effect of NB on flotation. It has been found that the presence of NB widens flotation particle size range and increased particle surface hydrophobic and improved froth flotation efficiency. Furthermore, negatively charged NB flotation with coagulation/flocculation process (PAC as coagulant) is concluded as to be a cost-effective treatment for chemical, mechanical polishing of wastewater. The conductivity experiments performed in the lab and the pilot scales conducted by reported an increase of 40% of wastewater clarification efficiency with a good removal of approximately 95% for the turbidity, total solids, and total silica. Moreover, the cost and performance of the NB flotation technology with coagulation/flocculation is found to be considerably lesser than the conventional processes. For a field scale application, the operation needs to be optimized to demonstrate the feasibility of the NB flotation technology.
Disinfection
Oxidation of pollutants and disinfection of pathogens using ozone is a promising method for treatment of drinking water and wastewater. Ozone gas bubbles have been applied for water treatment because of its powerful disinfection ability under low concentration and short contact time. Its disinfection efficiency increased its preference for the disinfection application of chemical resistance spore forming bacteria like Bacillus subtilis and Cryptosporidium parvum. Further improvement of its efficiency was achieved by applying MB. The observation of disinfection kinetic for E. coli by illustrated a faster reduction rate (approximately 99.99% of inactivation), a smaller tank size, and a lower ozone requirement for application of MBs in comparison to the conventional ozonation disinfection process. Another study on stopping multiplication of E. coli by cavitation of MB resulted in 75% reduction within 3 min at an energy input of 490 W/L. Similar results of the hydrodynamic cavitation reported in several studies convincingly prove the effectiveness of a non-reagent technique, such as MB and NB technologies, for water disinfection. The generation of OḢ and shock waves from collapsing MBs are discussed as the main cause of E. coli inactivation; however, the deactivation level of each influence factor still requires further research.
The inlet ozone concentration for the generation of MBs affected the removal of Bacillus subtilis spores, resulting in a greater log reduction at higher ozone concentration (i.e., 5-log reduction for 140 mg/L, 1.6-log reduction for 110 mg/L, and 0.3-log reduction for 40 mg/L with a contact time of 2 min). To achieve a 5-log reduction of Bacillus subtilis spores at a concentration of 40 mg/L of ozone, the time requirement is longer, found to be 5 min. This study concludes that high inlet-ozone concentration would enhance the log reduction of Bacillus subtilis because the dissolved zone rises during the disinfection. In addition, it has significant influence on reducing the cost of the process.
An analysis on deactivation for differing bacteria in a continuous test using the MBs indicated the effect of the bursting force of the bubble on the disinfection efficiency. The investigation revealed that E. coli was not detected after 30 min of MB generation. Moreover, the disinfection level of other bacteria is very high,> 90%. In addition, the optimum conditions for increasing the disinfection are reported to be smaller size and larger volume of the bubble and the bursting of the bubbles in the presence of bacteria at or near the water surface. In the application of NBs for bathing pools, observed a high prevention of pathogen growth. In this study, two circulation systems are applied to connect the bathing pool with a reservoir, and the range of the sizes of the NBs was 10–20 nm. It has been found that subsequent burst of the NBs would provide a greater disinfection and cleaning in both the bath and reservoir pools compared to that of the traditional ultrasonic vibrator.
The application of ozone MB is not only limited to disinfection as mentioned earlier. Recent researches revealed that reduction of bubble size also improved the oxidation of different pollutants by improving the formation of hydroxyl radical for advanced oxidation. In wastewater treatment, advanced oxidation process (AOPs) are known to have the capacity of converting most organic compounds in to carbon dioxide, water and mineral acids or salts. This mineralization process is achieved by an elevated amount of radicals generated in the system. Among AOPs O3 based systems have been showing better efficiency
in water treatment. In O3 based AOP processes the predominant radical species are the hydroxyl radicals (%OH). In such systems, the oxidation of any compound could occur via either through the oxidation of compounds via O3 molecule (direct reaction) or the oxidation through the reaction of the compound with the hydroxyl radicals formed from O3 (indirect reaction). Since the standard oxidation potential of O3 (2.07 V) is lower than the hydroxyl radical (2.80 V), the indirect oxidation of water pollutants via -OH generation is highly reactive and non-selective to almost all kinds of organic pollutants. Therefore the oxidation reaction with the pollutants is better accelerated by the indirect reactions [142]. But to improve the overall disinfection efficiency improving both reaction mechanisms is important. Different studies explained that ozonation and ozone based AOP (Advanced Oxidation Process) is affected by water properties like; pH, alkalinity, concentration and type of organic matter as well as temperature. The
pH of water is very important because the decomposition of O3 is accelerated by an increase in pH or concentration of OH−. In conventional bubbling systems generation of %OH is not detected in acidic pH. Similarly, studied the degradation of organic substances in winery wastewater, and observed that reduction of chemical oxygen demand (COD) under the action of O3 for acidic pH is negligible and more accelerated in neutral and alkaline pH by the fast -OH formation. Similar phenomenon has been observed by. But claimed that during the application of microbubbles the O3 concentration and -OH exposure in the acidic media is higher than at the basic pH. This result probably contradicts with the result of previous
researchers’ findings of less COD reduction in acidic pH. At this state it is recommended top further check the ambiguity in this aspect. But the existence of -OH radicals in the acidic medium could be explained
by the zeta-potential properties of MBs. It is known that microbubbles have a negative zeta-potential because of accumulation of OH− ions at their interface. As it is proposed by, the interior ozone gas of the microbubbles encounters these OH− ions surrounding the microbubbles while dissolving to the surrounding water. This accumulation of OH− ions acts almost as the same situation for ozone as being dissolved in a high pH even at low pH solutions. By this mechanism the MBs can improve the %OH leading to efficient degradation of organic matters even in acidic pH. Despite the advantages of MBs in increasing the concentration of O3 and -OH, which of course improves disinfection and waste degradation, studies are not yet done on the effect MBs on the formation of disinfection byproducts. Similar investigations are needed to confirm if the same situation happens for NBs. Further works are needed on the effect of micro NBs in O3 utilization efficiency, reactor optimization, and reduction of temperature effect as well as efficiency improvement test of specific pollutants for different pH.