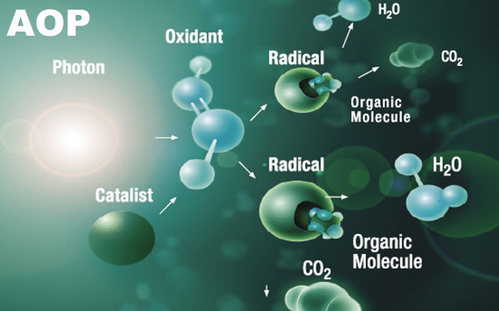
Advanced Oxidation Processes (AOP)
Advanced oxidation process es ( AOP s) have been defined by Glaze etal . as processes that “ involve the generation of hydroxyl radicals in sufficient quantity to effect water purification ” . AOPs are used to specifically generate OH ° . The OH ° have a higher oxidation potential (2.8 eV) than molecular ozone (2.07 eV) and can attack organic and inorganic molecules rapidly and non selectively. The most common processes are O 3 /H 2 O 2 , O 3 /UV and H 2 O 2 /UV. Research continues on AOP development as more cost – effective methods are sought. For example, using catalytic ozonation with dissolved or solid catalysts can reduce operating costs for pH adjustment since it is equally effective under both highly acidic and highly alkaline conditions or substituting solar energy for UV lamps can reduce energy costs. Each of these processes involves chemistry similar to that discussed above in the previous section. An overview of the reactions involved in the most common processes is presented below.
Micro Nano Bubbles Generator Pumps for Advanced oxidation Process with Ozone and ozonation
Ozone/Hydrogen Peroxide O3 / H2O
Hydrogen peroxide reacts with ozone when present as an anion, HO2 − . The reaction rate is dependent on the initial concentration of ozone/hydrogen peroxide.
The reaction of ozone with the undissociated hydrogen peroxide, which would lead to a loss of ozone and hydrogen peroxide, is negligible
The hydroperoxyl radical HO 2 ° and the ozonide anion O 3 ° − produced in above Equation then enter the chain reaction of the indirect pathway to produce OH °
Comparison of the initial reaction of ozone with HO 2 − ( k 9 = 2.2 × 10 6 M − 1 s − 1 ) and to that of ozone with OH − ( k 1 = 70 M − 1 s − 1 ) shows that in the O 3 /H 2 O 2 system the initiation step by OH − is negligible. Whenever the concentration of hydrogen peroxide is above 10 − 7 M and the pH – value less than 12, HO 2 − has a greater effect than OH − has on the decomposition rate of ozone in water.
The combination of Equations shows that two ozone molecules produce two OH °
Ozone/ UV – Radiation O 3 / UV
The advanced oxidation process with ozone and UV – radiation is initiated by the photolysis of ozone. The photo decomposition of ozone leads to hydrogen peroxide.
![]()
Guittoneau et al . confirmed that one mole of H 2 O 2 is formed from one mole of ozone at 254 nm and pH < 1.8, this ratio decreases with the increase of pH. This system contains three components to produce OH ° and/or to oxidize the pollutant for subsequent reactions:
⇒ UV – radiation
⇒ ozone
⇒ hydrogen peroxide
Therefore, all removal mechanisms should be considered when evaluating this AOP. Direct oxidation by hydrogen peroxide can usually be neglected under normal conditions (pH between 5 to 10 and ambient temperature). However, direct photolysis of the pollutant can occur if it absorbs the wavelength used. Depending on the conditions, direct and indirect ozone reactions with the pollutant are possible. In addition, combinations of the three components – ozone/ hydrogen peroxide or UV – radiation/hydrogen peroxide – also produce OH ° that contribute to the overall results.
Ultraviolet lamps must have a maximum radiation output at 254 nm for an efficient ozone photolysis.
Hydrogen Peroxide/ UV – Radiation UV / H 2 O 2
The direct photolysis of hydrogen peroxide leads to OH °
![]()
In addition, the ionized form of hydrogen peroxide HO 2 − , which is in acid – base equilibrium, also absorbs the wavelength 254 nm, and decomposes to produce one OH ° and an oxygen anion radical O °− , which can produce with H 2 O another OH °

Propagation
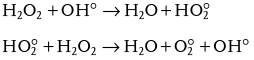
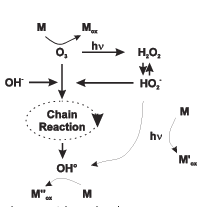
Above Figure gives an overview of the reaction involved in the AOPs. The reaction rate constant for all these reactions have been taken from several references and are presented to illustrate the various orders of magnitude. It is possible to find different values for one reaction. If model is going to be used to describe the measured data, the appropriate reaction constant has to be chosen to achieve the best fit.
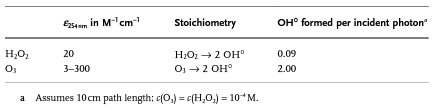
From the chemical point of view, the effect of O3/UV is comparable to that of O3/H2O2 if direct photolysis is negligible. Following table summarizes the chemistry involved in the generation of hydroxyle radicals from the described four processes.

In principle, the most direct method for generation of hydroxyl radicals is through the combination of hydrogen peroxide with UV. By the photosynthesis, 100% of hydrogen peroxide is transformed into OH0 . Nut the extinction coefficient of ozone at wave length of 25 nm is much higher than that of hydrogen peroxide.
![]() for O3
for O3
![]()
for H2O2
So the photolysis of ozone yields more hydroxyl radicals than that from hydrogen peroxide for the same energy input. Therefor, to achieve comparable treatment results, higher dosages of hydrogen peroxide or longer treatment time are necessary with UV/H2O2
This comparison shows the theoretical advantage of various reactions. In reality, a high production of hydroxyl radicals can lead to low reaction rate because the radicals recombine and are not useful for oxidation process. Also not considered are the effects of different inorganic and / or organic compounds in the water that can either accelerate or decelerate the reaction.
Further information concerning the parameters that influence the concentration of hydroxyl radicals will be given or discussed in next articles.